Thermodynamics: The Physics of Heat
Mr. Miller
Water, the Great Anomaly
Density, Convection, and the Saving of the Planet
The volume of most substances increases as the temperature increases. This is especially true when a substance changes phase, say, from a liquid to a gas or from a solid to a liquid. When the same amount of substance (the same mass) occupies a larger volume it becomes less dense. In fluids (which includes both liquids and gases), this means that the force of buoyancy will likely cause convection: movement will arise as the less dense substances rise to the top, while more dense substances descend.
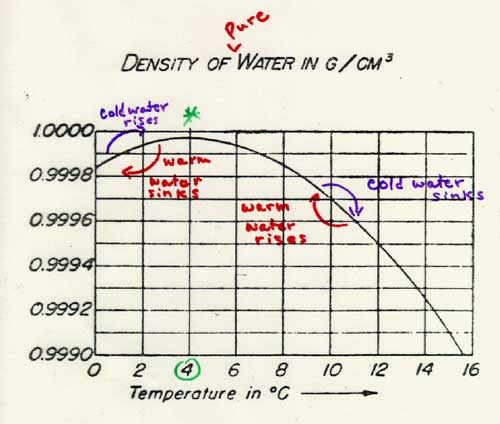
Water follows this general law up to a point, or perhaps we should say “down to a point.” Near its freezing point (which is also its melting point!) the behavior of water is anomalous. In fact, near its freezing/melting point, the density of water does not, like most liquids, decrease as the temperature rises. This means that water at these temperatures does not follow the rule that adding heat to the substance makes it expand! Instead, over a short range (from 0°C to 4°C), as the temperature of water increases, the water actually contracts and thus becomes less dense. Water has its greatest density at 4°C. As the temperature of the water rises above 4°C, water starts to expand like other substances. This strange behavior of water prevents ponds and lakes from freezing solid, and allows marine life to exist even when the surface of the water is covered with ice.
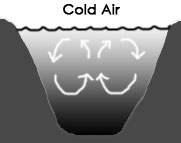
If the air above a freshwater pond is warmer than the water at the surface of the pond, the water gets colder as the depth increases, as seen in the diagram above, left. This is because the warmer water is less dense and therefore stratifies (forms layers) with the lease dense/warmest water at the top.
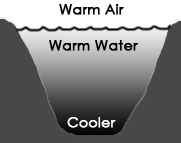
If the air at the surface is colder than the water at the surface, the water is chilled as its heat passes into the surrounding air, and it therefore becomes denser. This causes the top layer of water to then sink, as seen above in the diagram on the right. The falling cold water forces the warmer water just beneath it to the surface, where it contacts the air, gets chilled and therefore more dense, and sinks. This cycle causes a kind of circulation of the water called a convection cell, where density differences cause coherent cyclical movement in a fluid. If the air temperature is 4°C or above, this convection process will continue until ALL the water in the pond reaches the same temperature as the air (see diagram below left).


Once all the water in the pond is 4°C, the water stops the convection cycle. If the air outside becomes cooler than 4°C (say -10°C), then because the water is warmer than the air it will lose its heat to the air and become colder, according to our first rule of thermodynamics. But as the water cools below 4°C, it expands, and thus is less dense. This means that water between its freezing point (0°C) and 4°C will stay at the top of the water until it finally reaches 0°C, at which it turns to ice. A layer of ice thus forms on the surface of the water (see picture at right). If the air stays at -10°C, then there will be a temperature gradient in the ice, with -10°C ice at the surface and 0°C ice at the ice/water boundary. The water in contact with the ice is at 0°C, with a temperature gradient increasing to 4°C below, with all the rest of the water at 4°C. Thus the bulk of the pond does not freeze, and the fish and other life can survive.
Perhaps the following graph will help you understand this whole process: